

First Look: 2025 Perceptions & Insights Study
Discover key findings from our 2025 study with over 12,000 responses from around the world!

CISCRP’s Mobile Health Exhibit Brings Clinical Trial Education To Communities
Discover how a mobile exhibit is revolutionizing clinical trial education in underserved communities.
Empowering Informed Health Decisions: CISCRP’s Commitment to Health Literacy
Understand the importance of health literacy and the work CISCRP is doing to support research professionals and patient communities

From Compliance to Comprehension: Advancing Patient-Centered Informed Consent in Clinical Research
Explore how improving informed consent forms (ICFs) can enhance trial outcomes by improving participant understanding and retention.

Towards Electronic Product Information (ePI) That Meets the Needs of Everyone
This article discusses the evolution of electronic product information (ePI) and its potential to improve accessibility.
Considerations for the Use of AI in the Creation of Lay Summaries of Clinical Trial Results
This document is a product of collaboration between experts from over 15 organisations to explore how AI can be responsibly applied to LS development.

Journey to Better Health Mobile Exhibit | 2024 Baltimore
Our mobile exhibit hit the road for a second time in 2024 and had the opportunity to revisit Baltimore, strengthening our presence in this community.

Input Needed On Using AI To Create Lay Summaries Of Trial Results
The role AI can play in creating lay summaries of clinical trial results.

Public Discussion on Considerations for Use of AI in Lay Summary Creation
Join in on the growing topic of patient data protection and policies, to inform patients on the standard of patient data collection.

Mental Health Resources
Mental Health Resources

Pediatric & Caregiver Resources
Pediatric & Caregiver Resources

Diversity in Clinical Trials Package
Diversity in Clinical Trials Package

Finding Treatments Together Series: Mandarin
CISCRP brought several subject matter experts together to develop, design and release a 3-part, animated, live action video series.

General Clinical Research Overview: Mandarin
Brought to you by industry-leading subject matter experts.

Basics of Clinical Trial Participation: Mandarin
Brought to you by industry-leading subject matter experts.

The Clinical Research Team is Similar to a Sports Team: Mandarin
Brought to you by industry-leading subject matter experts.

Finding Treatments Together Series Spanish Language
CISCRP brought several subject matter experts together to develop, design and release a 3-part, animated, live action video series.

The Clinical Research Team is Similar to a Sports Team Spanish Language
Brought to you by industry-leading subject matter experts.

Basics of Clinical Trial Participation Spanish Language
Brought to you by industry-leading subject matter experts.

General Clinical Research Overview Spanish Language
Brought to you by industry-leading subject matter experts.
Diversity Video Collection
Our award-winning videos provide information about clinical research and how it can impact you, your family, and your community.

Diversity Content Package
The Importance of Diversity in Clinical Trials
Charts and Statistics
The charts and statistics below help you learn more about clinical trials.

Infographics
Infographics

Our Full Collection of Articles
News, information and articles

Research Services Special Reports
Perceptions & Insights Survey Special Reports from CISCRP

Finding Treatments Together Series
Brought to you by industry-leading subject matter experts.

Finding Treatments Together: The Clinical Research Team is similar to a Sports Team
Brought to you by industry-leading subject matter experts.

Finding Treatments Together: Basics of Clinical Trial Participation
Brought to you by industry-leading subject matter experts.

Finding Treatments Together
CISCRP has created a vast library of plain language clinical research content that is available to license, co-develop, customize, or sponsor.

Participant Bill of Rights
What you need to know before participating.

Should I Participate
This brochure includes information and questions that potential trial participants can ask to help them understand what being in a trial might mean.
Should My Child Participate
Deciding whether to enroll your child in a clinical trial can be complicated.

Clinical Research for Black and African American People
Educational brochure about clinical research for Black and African American communities.

Clinical Trials for Hispanics and Latinos
Educational brochure about clinical research for Hispanic and Latino communities.

A Guide to Costs and Payments in Clinical Trials
This brochure gives you the basic facts about costs and payments in clinical trials.

Common Myths About Clinical Trials
This brochure addresses some common myths about clinical trials.

The Importance of Diversity in Clinical Trials
Educational brochure about the importance of having diverse participants in clinical research studies.

Clinical Research Participation for LGBTQ+ Communities
CISCRP is committed to providing clear, unbiased, and culturally appropriate educational materials to engage and inform communities

Clinical Research for People in Asian Communities
CISCRP is committed to providing clear, unbiased, and culturally appropriate educational materials to engage and inform communities.

What is a Placebo?
If you or someone you know is considering taking part in a clinical trial, this brochure is for you.
How to Find a Clinical Trial
If you or someone you know is considering taking part in a clinical trial, this brochure is for you.

Clinical Research for American Indian and Alaskan Native Communities
CISCRP is committed to providing clear, unbiased, and culturally appropriate educational materials

A Guide to Oncology Clinical Trials
If you or someone you know is considering taking part in an oncology clinical trial, this brochure is for you.

Webinars
We work with organizations to develop highly informative webinars on a variety of topics for both professional and public audiences.

Finding Treatments Together: General Clinical Research Overview
Brought to you by industry-leading subject matter experts.
Building Patient-Centric Trials, Putting the Patient First
Building Patient-Centric Trials, Putting the Patient First
New Patient Engagement Insights from the 2019 CISCRP Perceptions & Insights Study
Discover the results of the 2019 Perceptions & Insights Study where over 12,450 people worldwide provided their opinions on clinical research.

Democratizing Clinical Research
Industry professionals discuss barriers to clinical trial participation and ways to address them.

AWARE for All- Northeast Webinar
This webinar shares information about the clinical trial process and how trial work form panelists and professionals in the Northeast region.

PLSP: Plain Language Summaries of Publication
PLSP: Plain Language Summaries of Publication

AWARE for All- Northwest Webinar
AWARE for All- Northwest Webinar

Understanding DCTs: Decentralized Clinical Trials
Learn what decentralized clinical trials are, how they work, and the importance of diversity in research participation in a 15-minute Flash webinar.

Improving Access to & Experiences of Transgender & Non-Binary Patients in Clinical Research
Improving Access to & Experiences of Transgender & Non-Binary Patients in Clinical Research

Rare Disease Clinical Trials
Rare Disease Clinical Trials

Supporting Caregivers in Clinical Research: Navigating the Pandemic
Supporting Caregivers in Clinical Research: Navigating the Pandemic

Findings from the CISCRP 2021 Perceptions & Insights Study
Learn about 2021 trends in clinical research awareness and perceptions, including shifts from past studies and patient engagement preferences.
AWARE for All- Atlanta Webinars
Watch the AWARE for All – Atlanta Webinar Series to learn about the clinical trial process and how trial work.

Straight Talk on Clinical Trials: Patient Perspectives on Clinical Trial Participation
Panelists discuss insights from a survey of patients on their perspectives of clinical trials and ways that sponsors can refine the process.

Voices From Within: Humanizing Clinical Research Data: Episode 1 – Patient Data Collection 101
In this webinar, panelists discuss the variety of ways in which patient data can be collected in clinical research.

Voices from Within: Humanizing Clinical Research Data: Episode 3- Conversations on Clinical Trials
Join Curebase's discussion speaking with a clinical trial participant and their experience in CISCRP's webinar.

Voices from Within: Humanizing Clinical Research Data: Episode 2- Conversations on DCTs: Data Privacy
Join in on the growing topic of patient data protection and policies, to inform patients on the standard of patient data collection.
The Clinical Trial Challenge: Boosting Clinical Trial Appeal in Patient Communities – Part 2
Panelists discuss the results of a survey exploring factors that could make participation of interest to patients and family caregivers.

The Clinical Trial Challenge: Boosting Clinical Trial Appeal in Patient Communities – Part 1
Pam Cusick and patient advocate Grace Charrier discuss a RPV survey exploring factors that could increase clinical trial participation.

Voices Yet to Be Heard: Including People with Disabilities in Clinical Research
Panelists share their experiences participating in clinical research, accessibility challenges, and suggestions for improvements.

Working Towards a More Inclusive Environment: Transgender & Non-Binary Participants in Clinical Research
Learn insights on significant barriers that trans and non-binary participants face and how to create more diverse and inclusive clinical trials.

Insights on Developing an Impactful DEI Video
Hear from our panelists who share insights gained while developing CISCRP’s video, The Importance of Diversity in Clinical Trials.

Webinar on the 2023 Perceptions & Insights Study
Discover the results from our latest 2023 study, with over 12K responses from around the world!

Findings from a Long-Term Patient Engagement Model
Clinical Researcher: The Authority in Ethical, Responsible Clinical Research

Meeting UK IRB/EC Expectations for Patient Review of Research Participant Information
Review key elements of these expectations and practical considerations for meeting them.

Videos
Our award-winning videos provide information about clinical research and how it can impact you, your family, and your community.

CISCRP Partners with SubjectWell on Survey
CISCRP partnered with SubjectWell to review how race and gender impact clinical trial participation, during the COVID-19 pandemic.

Journey to Better Health Mobile Exhibit | 2023 Events
Journey to Better Health is a mobile exhibit that showcases clinical research information to local communities

Latest CISCRP Patient Survey Reveals Diversity Gaps, Yields 5 Tips For Improvement
More effort is needed to understand the views of underserved groups, such as ethnic and racial minorities, toward clinical research

Latest CISCRP Patient Survey Reveals Diversity Gaps, Yields 5 Tips For Improvement
More effort is needed to understand the views of underserved groups

Plain Language Summary Publication of Key Results from Bayer’s Phase 3 ARAMIS Trial Published in Future Oncology
CISCRP and Oxford PharmaGenesis worked with Bayer to write a PLSP of the 2020 New England Journal of Medicine article on the ARAMIS trial.

Round-Up: Highlighting Recent Health Literacy Educational Materials
Round-Up: Highlighting Recent Health Literacy Educational Materials

A “Day in the Life” of a Medical Writer at CISCRP
It is Monday morning at the CISCRP office, and I open my computer a few minutes

Community Trust: The Foundation for Fostering Diversity in Clinical Trials
Racial and ethnic minority populations have historically been underrepresented in clinical trials.

Plain Language Protocol Synopsis 101
Should Sponsors Add a Plain Language Protocol Synopsis to Future Clinical Trial Applications?

Community Trust: The Foundation for Fostering Diversity in Clinical Trials
Featured Article in our October 2023 Patient Diversity Campaign

CISCRP’s Finding Treatments Together Brochure for LGBTQ+ Communities
Our Approach to Codeveloping an Educational Resource for the LGBTQ+ Community

The Importance of Transgender and Non-Binary Inclusion in Clinical Research
At CISCRP, we value the importance of engaging and informing the groups that are underrepresented in clinical trials.

The Importance of Transgender and Non-Binary Inclusion in Clinical Research
Recapping the webinar in collaboration with Clario
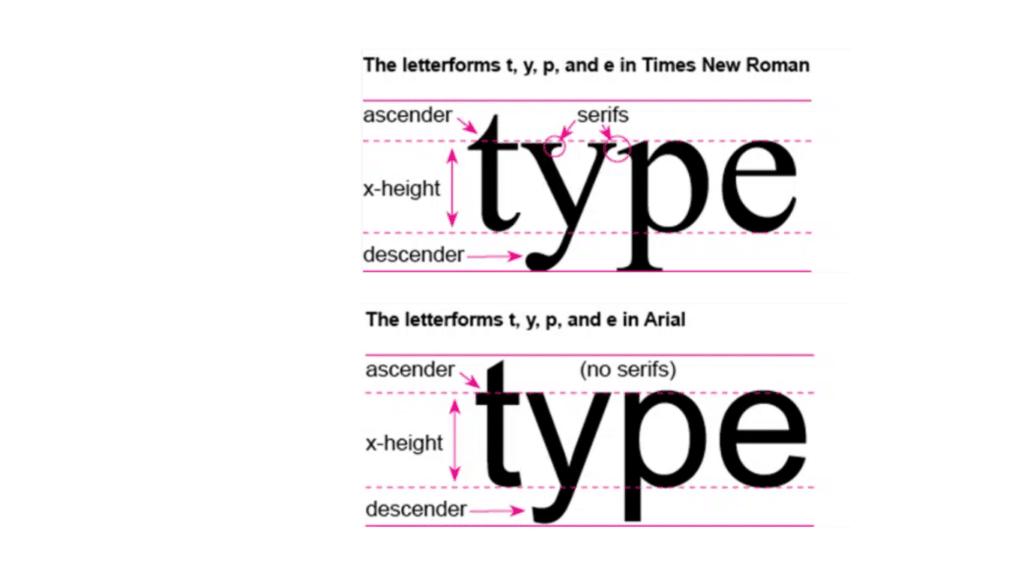
Best Practices in Design for Clinical Trial Communications: The Mysterious Art of Typography
CISCRP produces a wide range of patient-friendly materials, such as informed consent forms, brochures, and trial summaries.

Why Diversity in Clinical Trials Matters – May 2023 Patient Diversity Campaign
Article from our 2023 May Patient Diversity Campaign

Why Diversity in Clinical Trials Matters – May 2023 Patient Diversity Campaign
Clinical trials are a critical part of the research process for new medicines and vaccines

The Importance of Patient Engagement and its Role in Clinical Trials
The Importance of Patient Engagement and its Role in Clinical Trials

Behind the Scenes of Health Literacy at the Movies
Learn about the infographic that contains an exercise to help readers brush up on their health literacy knowledge.

How CISCRP Made Tools for Informed Decision Making
How CISCRP communicates clinical research information to the public

Improving Accessibility in Clinical Trials
How to improve accessibility in clinical research

The Importance of Diversity in Clinical Trials
To develop therapies and treatments for everyone, it is important that clinical trial participants come from diverse backgrounds and identities.

Voices from Within: Conversations on DCTs & Data Privacy
In Episode Two of Voices from Within: Humanizing Clinical Research Data, Curebase shares a comprehensive overview of how patient data is collected.

Clinical Trial Participants’ Selfless Gift to Medicine
"The Gift of Participation" by CISCRP Founder Ken Getz

Improving Health Outcomes Through Equity and Access
Biogen discusses their efforts to improve health outcomes through equity and access.

The Couple That’s Empowering Communities of Color to Participate in Parkinson’s Disease Clinical Trials
Discover the Parkinson’s disease diagnosis that spurred an empowering journey Denise is still on today.

Why Participation in Clinical Trials Is a Must for Hispanics
Clinical trials need ethnically diverse participants so scientists can develop a greater understanding of how diseases impact different people.

Press Release: CISCRP Receives Innovation Award from FDA’s Office of Minority Health and Health Equity for Research Project on Pilot Mobile Community Engagement Initiative
Initiative Aims to Bring Clinical Research Education and Resources to Medically Underserved Communities

DEI Series — Educating Clinical Research Practitioners Through Video
CISCRP partnered with WCG to create a video for researchers and study staff that emphasizes the importance of diversity in clinical trials.

Improving LGBTQ+ Inclusivity in Ovarian Cancer Care
From an ovarian cancer awareness perspective, there are specific messages for the LGBTQ+ community that need to be communicated.

Put Your Customers in Focus With Patient Experience Mapping
What can healthcare organizations do to maintain resilience while ensuring their products make it to those who need them most?

Broadening the Lens of Diversity for More Inclusion in Clinical Research
Ensuring diversity, equity and inclusion means more study participants, thorough science, and predictable outcomes for all potential patients.

New Data Reveals Decentralized Clinical Trials Yield Better Clinical Trial ROI
CISCRP Founder Ken Getz discusses decentralized clinical trials (DCTs) and how their value can be measured

Broadening the Lens of Diversity for More Inclusion in Clinical Research
For many, clinical trials offer free or low-cost access to testing, care, and cutting-edge treatments that are simply not accessible or available.
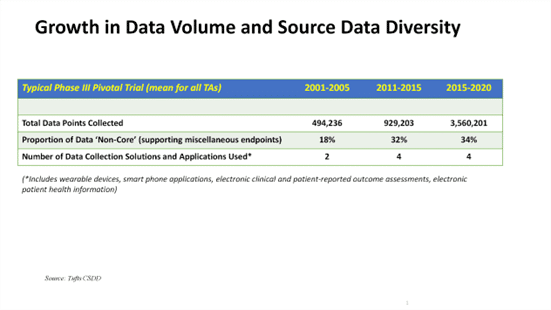
Patient Data Collection 101: Curebase FLASH Webinar Overview
There are a variety of ways in which patient data can be collected in clinical research.

Clinical Trial Care and Compensation
From "The Gift of Participation" by Ken Getz, Founder & Board Chair, CISCRP

Addressing Barriers to Clinical Trial Enrollment
Addressing barriers to clinical trial enrollment

Improving Representation in Breast Cancer Clinical Trials & Developing Better Medicines for All
Article from our 2021 May Patient Diversity Campaign

Recognizing the Gift of Participation
An excerpt from "The Gift of Participation" by Ken Getz, Founder & Board Chair, CISCRP

Press Release: Center for Information and Study on Clinical Research Participation Receives National Award from Health Industry Leaders
Healthcare Leadership Council Honors CISCRP for Promoting Engagement and Partnership Between Clinical Research Professionals, Patients, and the Public

Press Release: From Subject to Partner Publication
CISCRP Launches New Publication Marking the 20th Anniversary of TIME Magazine’s Clinical Research Issue

The Patient Journey in Clinical Research: Insights & Observations to Scale Engagement
Webinar panelists discuss Rare Patient Voice and Informa Pharma Intelligence's survey results about patient perspectives in clinical research

No “Kidding”: Using Health Literacy to Communicate Clearly About Pediatric Trials
Using Health Literacy to Communicate Clearly About Pediatric Trials

Meeting the EU Regulation: Plain Language Summaries & Protocol Synopses
Meeting the EU Regulation: Plain Language Summaries & Protocol Synopses

Jeh Jeh Pruitt, A New Advocate for Clinical Trials: CISCRP’s AWARE for All Program Featured by CSL Behring
Jeh Jeh Pruitt at CISCRP's Journey to Better Health - AWARE for All event in Birmingham, Alabama

Are Scientists On the Verge of an Alzheimer’s Cure?
Scientists are working towards a cure for Alzheimer's, with the help of clinical trial participants

Videos in Health Literacy
Addressing the effectiveness of videos in clinical research education

How African American Men Can Beat the Odds Against Prostate Cancer
What African American men can do to fight prostate cancer

Health Literacy: Making Content Clear, Engaging, and Appropriate for Patients and the Public
What is healthy literacy and what is CISCRP's Health Literacy team doing?
CISCRP’s Newly Launched Educational Brochure Initiative
Educational materials from CISCRP's Health Communication Services team

Key Insights Into Clinical Research Perceptions Among Parents and Children
Findings from CISCRP's online survey about clinical research perceptions among parents and children

The Priceless Gift of Clinical Trial Participation
Ken Getz, Founder of CISCRP, discusses the importance of clinical research participation

Expanding Clinical Trial Access for Women Living With HIV
Merck is working with community outreach groups and women living with HIV to understand the burden of the disease and what solutions might help

Rare Disease Clinical Trials: How to Prepare for When the Clinical Trial Ends
Questions to ask the clinical research study staff before the trial ends

Participating in a Clinical Trial: Building A Support Network
From “The Gift of Participation” by Ken Getz, Founder & Board Chair, CISCRP

An Inside Look at Advocating for Yourself or Your Child in a Clinical Trial
How to advocate for yourself or your child in a clinical trial, topic featured in a CISCRP Flash webinar

Adolescents in Clinical Trials & the RACE for Children Act
Learn about the future of pediatric oncology development, treatment options, and adolescents treatment in light of the RACE for Children Act.

The Importance of Telehealth for Rare Diseases
Healthcare must reflect all of the opportunities of telehealth, but especially so for those suffering from rare diseases.

What Does a Caregiver Need?
Dealing with significant health challenges requires expert medical care and the support of committed caregivers.

Boosting Clinical Trial Appeal in Patient Communities Part 2: Rare Patient Voice FLASH Webinar Overview
Ultimately, no one wants to feel alone during their clinical trial experience.

Clinical Trial Participants Are Changing Lives
Article from 2020 Clinical Trials Supplement, USA Today

Rare Disease Clinical Trials: Being Informed about Clinical Research
CISCRP hosted a 3-part webinar series titled “Navigating Rare Disease and Clinical Research: Every Patient Matters”.

Results From The 2021 CISCRP Perceptions & Insights Study Coming Soon
Preview of findings from CISCRP's 2021 global Perceptions & Insights Study

Clinical Trials: Every Person Can Play A Powerful Role
Marie-Pierre Hellio La Graverand, M.D., Ph.D, Pfizer, gives her three takeways she’d want people to have about clinical trials

Rare Disease Clinical Trials Series
Join in on the growing topic of patient data protection and policies, to inform patients on the standard of patient data collection.

PLSP: The Results Of The DESTINY-Breast Cancer01 Clinical Trial Published In Future Oncology
CISCRP's plain language summary publication from the results of the DESTINY-Breast01 clinical trial

Why Clinical Trial Diversity Is Key To Increasing Access To Routine Care And Innovative Treatment Options
Article by Luther T. Clark, MD, Deputy Chief Patient Officer, Merck

Women in Clinical Trials
From “The Gift of Participation” by Ken Getz, Founder & Board Chair, CISCRP

Why Clinical Trials Are Conducted
An excerpt from CISCRP Founder Ken Getz' "The Gift of Participation"

CISCRP & Partners’ PLSP on Breast Cancer Study Published in Future Oncology
CISCRP's plain language summary publication (PLSP) of the results of the DESTINY-Breast01 clinical study
AWARE for All- Midwest Webinars
AWARE for All- Midwest Webinars

Out of the Dark: A Journey Through Postpartum Depression, Part 1
CISCRP Webinar | Out of the Dark: A Journey Through Postpartum Depression, Part 1

Out of the Dark: A Journey Through Postpartum Depression, Part 2
CISCRP Webinar | Out of the Dark: A Journey Through Postpartum Depression

Pros & Cons of DCTs & Virtual Clinical Trials
From "The Gift of Participation" by Ken Getz, Founder & Board Chair, CISCRP

Clinical Studies Are Building a Brighter Future for People With Deafness
A mother and son share their experiences at the forefront of cochlear implant clinical research.

The Importance of Clinical Research in Underserved Communities
The importance of clinical research is widely recognized, and while many decide to participate in clinical trials, there is a lack of representation.
Recognizing Research Professionals Who Participated in COVID-19 Trials
This video recognizes research professionals in the clinical research industry who participated in COVID-19 trials.
CISCRP Provides Plain Language Clinical Trial Communication Services to Support Operation Warp Speed Vaccine Sponsors
CISCRP Provides Plain Language Clinical Trial Communication Services to Support Operation Warp Speed Vaccine Sponsors
2020 Pediatric Perceptions & Insights Study Yields Important Insights on How to Enhance Pediatric Clinical Trial Participation
Survey Conducted by CISCRP about the perceptions of pediatric trials

Parents & Children Share Perceptions & Experiences with Clinical Research: Survey
Results from CISCRP's online survey about parents' and their children's perceptions of pediatric trials

CISCRP Launches Education Video Campaign on Clinical Trial Participation During the Pandemic
CISCRP's educational video about clinical research
CISCRP Licenses Brochures to Ukrainian Association for Clinical Research (UACR)
CISCRP licensed brochures for translation into Ukrainian by UACR for their members and general public to learn more about clinical research

CISCRP Explores the Impact of COVID-19 on Clinical Research
CISCRP survey assesses the impact of the pandemic on clinical research perceptions and experiences

CISCRP Announces 2nd Annual Virtual Fitness Challenge to Recognize Medical Heroes
Encouraging the clinical research community to come together across the globe to honor healthcare professionals, researchers, and study volunteers

Building A Clinical Trials Website that Engages Patients & the Public
Building A Clinical Trials Website that Engages Patients & the Public

Journey to Better Health Mobile Exhibit 2018
Journey to Better Health mobile exhibit is our traveling educational exhibit with the goal to raise awareness about clinical research.