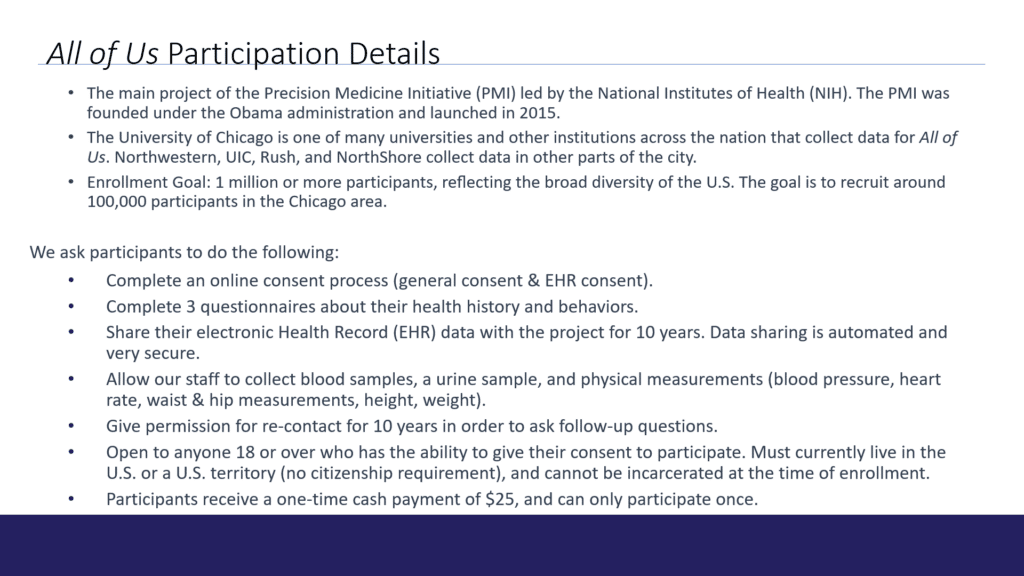
As the effective date of the EU regulation approaches, research sponsors are establishing programs that meet the requirement to provide lay language summaries of their clinical trials’ results.
To address the most pressing challenges of implementing lay summary and patient engagement programs, CISCRP held its Inaugural Lay Summaries User Group Meeting on Wednesday, May 16, 2018, at the EMD-Serono campus in Rockland, MA. This unprecedented workshop-style discussion included representatives from AstraZeneca, Biogen, CSL Behring, EMD Serono, Janssen, Novartis, WIRB-Copernicus Group, and CISCRP’s Communicating Trial Results.
CISCRP’s Communicating Trials Results (CTR) service directly supports the development of lay language summary programs. Our dedicated team of lay language experts, medical and legal writers, and translators work directly with some of the top pharmaceutical companies in the world to provide their clinical trial participants with plain language trial results. Since 2012, our CTR team has shipped over 50,000 summaries to trial sites all over the globe.
The inaugural meeting was an invaluable opportunity for CISCRP to meaningfully engage and collaborate with our most active sponsors. The meeting highlighted the need for increased cross-collaboration with our sponsors to further refine the best practices that are established and shared within CISCRP’s program. The shared passion and enthusiasm among attendees for patient engagement and education was truly inspiring.
Robert Janiak, Head of Clinical Trial Transparency at Merck, kicked off the discussion with opening remarks. He was followed by CISCRP Founder and Board Chair Ken Getz, who spoke to the importance of engaging with CISCRP’s program sponsors to build rapport, communicate ideas, and share each other’s enthusiasm and passion for patient advocacy and education.
The role Institutional Review Boards (IRBs) play in plain language disclosure was at the top of the agenda. Change requests from IRBs raise concerns around data consistency across document versions, while IRB requests to submit lay summaries for any purpose beyond awareness can extend an already limited timeframe for submission to the forthcoming EU portal and database. Although several organizations provide guidance for publishing trial results summaries (SAHCRP in the US; HRA in the UK; MRCT; and Transcelerate), sponsors struggle with a lack of consistency across different guidelines.
Lindsay McNair, Chief Medical Officer at WIRB-Copernicus Group, was on hand to help understand the IRB perspective and address concerns around submission of the trial results summary. Her insights helped the group identify best practices to navigate the situation, suggesting that proactive communication and education is needed.
As a result of this discussion, CISCRP and WIRB-Copernicus Group will collaborate to develop template language to use when communicating with IRBs. We are hopeful this language will facilitate a unified approach to IRB submission of trial results summaries throughout the industry while also acknowledging existing recommendations.
Other forum topics included Trial Result Summary Content and Formatting, Process Considerations for Implementing Successful Programs, and What’s Next for Patient Engagement? We explored these and many more topics in detail over the course of our discussion.
We are very grateful to our friends at EMD-Serono for hosting and to all of our sponsor representatives who shared their valuable insights and first hand experiences. With a growing number of sponsors engaging with CISCRP’s program, we look forward to seeing many new faces at the next meeting!
Written by Robert Mills, Project Manager for Communicating Trial Results